Annovis Bio: A Close Look At Their Parkinson’s Trial Data (NYSE:ANVS)

Jonathan Knowles
Annovis Bio’s Parkinson’s Trial Announcement
On Tuesday, July 2, Annovis Bio, Inc. (NYSE:ANVS) announced data from their long-expected phase 3 Parkinson’s Disease (PD) trial.
The market responded dramatically and positively to the news, which resulted in a 76% rise in the stock price, to close at $9.28 on July 2 from $5.27 the previous day.
Was this price action on July 2 simply the crazy volatility of a micro-cap R&D biotech stock with a high short interest, or a well-justified response to a significant phase 3 PD trial readout?
To answer this question, let’s dive into the PR for a close look at the four summary statements and their corresponding data.
Statement #1
Buntanetap showed dose-dependent and statistically significant improvements in cognition in the overall enrolled PD population. Parkinson’s patients with substantial cognitive decline exhibited a very pronounced improvement.
(Bold added by the author for emphasis).
This statement summarized what was presented in Fig 1 (see below).
Fig 1 (ANVS PR)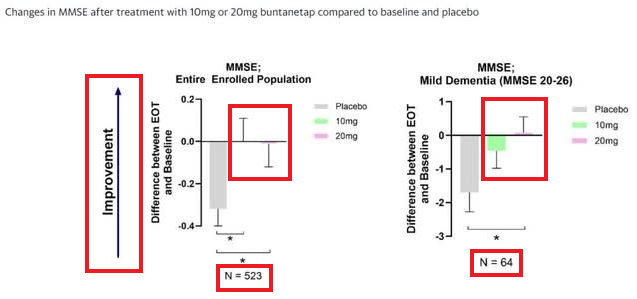
(Red boxes added by the author; Improvement direction is UP).
Three things to note here:
- The “dose-dependent and statistically significant improvement improvements in cognition in the enrolled PD population” (n=523) is an inverse one, i.e., 10mg improved more than 20mg.
- This inverse “dose-dependent” improvement in cognition occurs in the overall enrolled patients (n=523), but is reversed in the “Mild Dementia MMSE 20-26” subgroup, where the 10mg group has no statistically significant improvement.
- MMSE improvement data is the only data reported from the entire enrolled patients (n=523) in the PR.
Statement #2
Buntanetap showed statistical improvement in the MDS-UPDRS Part II, Part III, Part II+III, and Total scores in Parkinson’s patients with a >3-year diagnosis.
(Bold added by author for emphasis).
Statement #2 summarizes what was presented in Fig 2 (see below).
Two things to note here:
- These MDS-UPDRS data are from a subgroup, “HY 1&2 MH>3yrs” patients, (n=158), and not from the entire group of enrolled patients (n=523).
- Only the 20mg-treated subgroup had statistically significant improvement over the placebo (n=158) in all four data sets, as indicated by * in Fig 2 above.
Statement #3
Buntanetap showed the same statistical improvement in MDS-UPDRS Part II, Part III, Part II+III, and Total scores in Parkinson’s patients with Postural Instability and Gait Difficulties (PIGD).
(Bold added by author for emphasis).
Statement #3 summarizes what was presented in Fig 3 (see below).
Two things to note here:
- This MDS-UPDRS data is also from a “PIGD” subgroup (n=98*) and not from the entire group of enrolled patients (n=523).*Note: “PIGD” subgroup number was not disclosed in the PR, but was in the AH conference call presentation.
- Only the 20mg-treated subgroup had statistically significant improvement over the placebo (n=158) in all four measurements, as indicated by * or *** in Fig 3 above, vs. 10mg, which had mixed results, e.g., not significant in part III and Total [Top and bottom charts on the right side].
Statement #4
Buntanetap’s activity resulted in statistically significant improvements in all primary and secondary endpoints in the specified populations as well as in cognition.
(Bold added by author for emphasis).
This is perhaps the most important summary statement, as it summarizes the previous three, or the trial results as disclosed in the PR. Therefore, in my opinion, it’s prudent to assess its accuracy.
Regarding the trial’s endpoints, I’ve compiled the table below from the trial protocol found on the clinicaltrials.gov site.
Primary endpoints |
| Change From Baseline to Month 6 in MDS-UPDRS Part II (OFF-state) |
| Safety and tolerability |
Secondary endpoints |
| Change from Baseline to Month 6 in the sum of MDS-UPDRS Part II+III (OFF-state) |
| Change from Baseline to Month 6 in the MDS-UPDRS Part III (OFF-state) |
| Change From Baseline to Month 6 in The Sum of MDS-UPDRS Total Score (OFF-state) |
| Percentage of Responders with “Much Improved” or “Very Much Improved” on Participant Global Impression of Change (PGIC) (ON-state) |
| Change From Baseline to Month 6 on Change on Clinical Global Impression of Severity (CGIS) (OFF-state) |
(Compiled by author)
As can be seen above, there are five secondary endpoints listed in the protocol, per clinicaltrials.gov site.
In the PR, ANVS only reports the first three secondary endpoints, i.e., MDS-UPDRS Part II, Part II & III, Part III, Total Score (shown in Fig 2 & 3), and not the last two endpoints, i.e., PGIC & CGIS data
In terms of the word “specified“, the protocol specifies no such subgroups.
In other words, these are the “subgroups” formed from a post-hoc analysis, i.e., not pre-specified, per protocol subgroups.
The difference between pre-specified subgroups and post-hoc-analysis-derived subgroups is that the latter is specified after the un-blinding, i.e., trying to find a plausible criteria that might explain why the best-[drug-treated] responders have the best response.
In my opinion, these post-hoc-derived criteria, while looking promising, are far from reliable, and should not be confused with the original data, produced per protocol.
In summary: While statement #4 sounds very positive, one should note that:
- MMSE improvement (cognitive benefit) is not among this trial’s specified efficacy endpoints.
- Only 20mg [vs. 10mg and 20mg both] was statistically significant in all the MDS-UPDRS results.
- Only three out of five per protocol secondary endpoints from subgroups are reported.
- The “specified” subgroups are post-hoc analysis-derived subgroups [vs. per protocol subgroups].
Annovis Bio’s AH Conference Call
In their AH conference call, ANVS disclosed the MDS-UPDRS II (trial’s primary efficacy endpoint) and MDS-UPDRS III results (one of 5 secondary endpoints) from the ITT (intend-to-treat) patients (n=523).
As can be seen above, in the ITT patients (n=523), the drug has shown no effect in MDS-UPDRS II and III scores, i.e., both treatment groups showed no statistically significant improvement over the placebo, as measured by MDS-UPDRS Part II and Part III.
In other words, the trial has not met either its primary or one key secondary efficacy endpoint in the enrolled intend-to-treat patients (n=523).
Financials
According to the May 10-Q filing (page 6, see below), the company has a $3.1M cash runway, and a net quarterly burn of $1.1M, as of March 31, 2024.
ANVS 10-Q Form, May 2024
The company also has an ELOC in place, in which they can sell 2 million shares to raise additional funding.
As ANVS plans to conduct more phase 3 trials in AD and PD (that are larger and longer), they will no doubt need to raise significant additional funding in the near term.
Discussion & Conclusion
I covered ANVS’ Alzheimer Disease (AD) trial data recently and gave a “Strong Sell” rating, for the reasons discussed in that article.
After this PD trial data, my estimate for the company’s long-term prospects remains bearish.
While the announcement may seem positive, this PD trial did not meet its pre-specified (primary & one reported secondary) efficacy endpoints in the overall ITT patients, measured by MDS-UPDRS part 2 and part 3.
Even if the MMSE (cognitive benefit) results are statistically significant in ITT patients, I doubt that the MMSE data [not a pre-specified efficacy endpoint] alone will be sufficient for a filing in Parkinson’s Disease and/or to secure an approval.
Similar to their AD program, the company plans to conduct further trials in PD, perhaps focusing on the “subgroup” patients whom they identified as most likely to respond to the drug.
I, however, am skeptical of such an approach [studying post-hoc subgroups as the next ITT] and find it an unreliable method for securing future success.
Therefore, as an investment opportunity, I’ll choose a SELL rating, as I’m bearish regarding ANVS’ long-term prospects.
Risks
Risks associated with a bearish thesis include but are not limited to:
- Dramatic price actions to the upside, e.g., a 76% rise in share price, from $5.27 previous close to $9.28 on July 2, after the PD trial data announcement.
- The company secures significant non-dilutive funding through grants or partnerships.
- ANVS’ future phase 3 trials in AD & PD meet pre-specified endpoints successfully in the ITT patients.
- Buntanetap is ultimately successfully developed/approved for market in AD or PD or both.
Thank you for reading. Hopefully, this article is clear and helpful in your research into this company. All the best!
Editor’s Note: This article covers one or more microcap stocks. Please be aware of the risks associated with these stocks.








